Study suggests approach for treating rare disorder
At a Glance
- Using 3D brain tissue structures called organoids, researchers tested a potential new therapy for a rare disorder called Timothy syndrome.
- The results suggest that antisense oligonucleotides could form the basis for new treatment approaches for the disorder.
Research into rare diseases and disorders is hampered by the limited number of people available to participate in studies. Many rare diseases and disorders also lack animal models that can help scientists perform early testing of new treatments.
One such rare disorder is Timothy syndrome. Problems with a single gene, called CACNA1C, cause this condition. CACNA1C codes for structures in cells called calcium channels. These help control signals throughout the brain and body. People with Timothy syndrome live with a range of severe symptoms including autism, epilepsy, and interruptions in the heart’s normal electrical activity.
After the CACNA1C gene is transcribed into RNA, the RNA can be spliced in different ways to make different versions of the protein. Normally, splicing changes cause one version of the protein to be produced during development, and then another later on. In Timothy syndrome, the first version continues to be produced instead. As a result, calcium channels remain overactive, causing abnormal electrical activity in the body’s cells.
Researchers have been developing compounds called antisense oligonucleotides, or ASOs, to treat some rare genetic disorders. ASOs bind to small portions of RNA to affect how the RNA is processed. In a new study funded in part by NIH, a research team led by Dr. Sergiu Paşca from Stanford University tested whether ASOs could block the changes in RNA that cause Timothy syndrome.
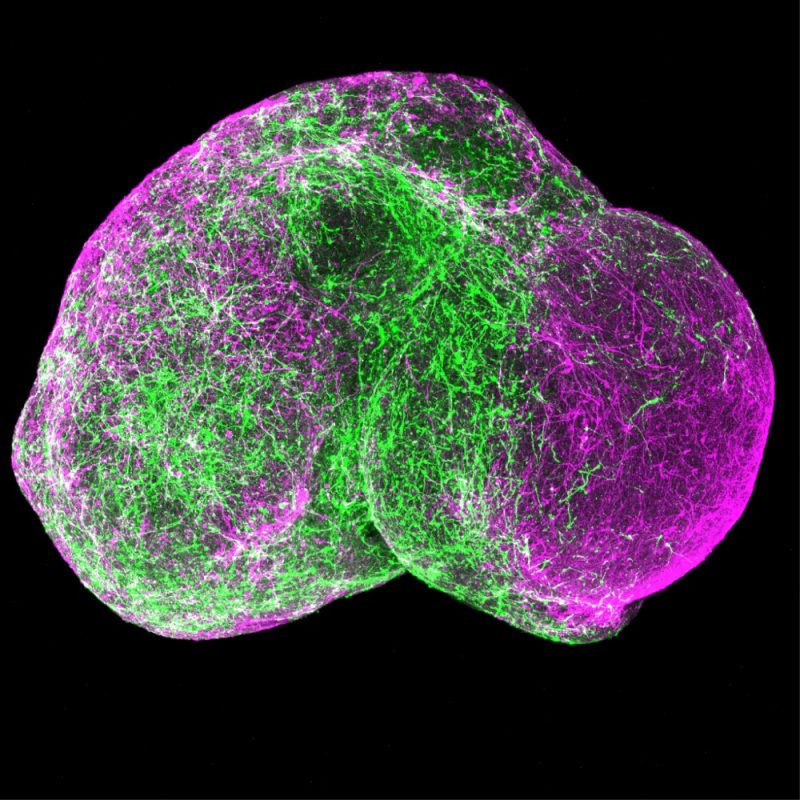
The team first grew 3D human brain tissue structures called organoids from stem cells donated by people with Timothy syndrome. The study was published on April 25, 2024, in Nature.
The researchers developed several ASOs that, in organoids grown to mimic the brain’s cerebral cortex, reduced the abnormal RNA splicing. Adding the two most promising ASOs to organoids derived from people with Timothy syndrome restored the functioning of calcium channels to normal levels.
To see whether the treatment could restore normal cell movements during brain development, the team created more complex structures called assembloids. These were made from different organoids fused together to mimic the human forebrain. Treating these assembloids with ASOs restored the normal movement of brain cells during development.
Timothy syndrome manifests during early development. To test how the ASOs might work in the developing brain, the researchers transplanted the human-derived organoids into the brains of newly born rats. The organoids integrated successfully into the growing brain tissue. When the researchers injected the rats with one of the ASOs, production of the abnormally spliced RNA was reduced. Calcium channels in the resulting cells worked more normally, and the cells looked normal under the microscope.
“Our study showed that we can correct cellular deficits associated with Timothy syndrome,” Paşca says. “We are now actively working towards translating these findings into the clinic, bringing hope that one day we may have an effective treatment for this devastating neurodevelopmental disorder.”
Notably, the approach used in this study has potential for studying new treatments for other rare genetic disorders as well. Many challenges, however, still need to be solved before this technology could be used in the clinic.
References: Antisense oligonucleotide therapeutic approach for Timothy syndrome. Chen X, Birey F, Li MY, Revah O, Levy R, Thete MV, Reis N, Kaganovsky K, Onesto M, Sakai N, Hudacova Z, Hao J, Meng X, Nishino S, Huguenard J, Pașca SP. Nature. 2024 Apr;628(8009):818-825. doi: 10.1038/s41586-024-07310-6. Epub 2024 Apr 24. PMID: 38658687.
Funding: NIH’s National Institute of Mental Health (NIMH); Stanford University; Autism Speaks, Inc.; Kwan Funds; Senkut Funds; Coates Foundation; Ludwig Family Foundation; Alfred E. Mann Foundation; New York Stem Cell Foundation; Chan Zuckerberg Initiative.








