Genome editing restores hearing in mice
At a Glance
- Scientists developed a genome editing method to restore hearing in adult mice with a rare type of genetic hearing loss.
- With additional study, this type of approach might one day help to reverse some types of inherited hearing loss in people.
For over half of children born with hearing loss, a single genetic abnormality is to blame. This is called inherited deafness, or genetic deafness. Hearing loss can impair a child’s speech, language, and social skills. Early interventions can often help. But no medications or other treatments have yet been approved for slowing or reversing progression of inherited deafness.
Scientists have been searching for ways to restore hearing via gene editing, which entails modifying the genetic sequence. Some have successfully improved hearing in mice with different types of hearing loss. But to date, such studies had only been successful in newborn mice, which still have immature structures in their inner ear. These structures continue to change in shape and function until adulthood. In contrast, the inner ear is fully developed in newborn humans.
A research team led by Dr. Zheng-Yi Chen at Mass Eye and Ear in Boston reasoned that developing a gene editing approach that works in adult mice, with a fully mature inner ear, might make it more likely to also work in humans. Their study appeared on July 10, 2024, in Science Translational Medicine.
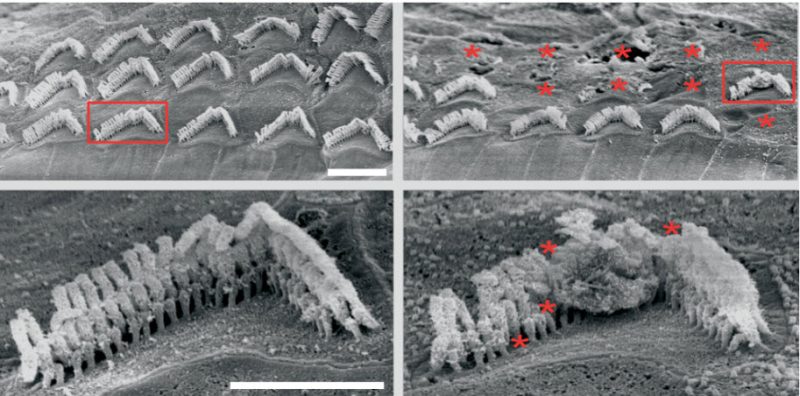
The scientists created a mouse model with a specific mutation in the microRNA-96 (MIR96) gene. MIR96 helps to regulate gene activity in the ear’s tiny hair cells, which transform sound into electrical signals. Mice with the mutation lose their hair cells and develop complete hearing loss in the high frequencies four weeks after birth. In humans, the same mutation causes a rare type of genetic hearing loss called autosomal dominant deafness-50 (DFNA50). People born with DFNA50 tend to have progressive hearing loss beginning in their teens.
The research team developed a CRISPR-Cas9 gene editing system that could disable faulty versions of the MIR96 gene in living mice. A harmless adeno-associated virus (AAV) was used to deliver the system into cells. This was injected into the inner ear of some mutant mice at 3 weeks of age, before the onset of hearing loss. Other mice received the injection at 6 weeks, as adults with hearing loss. Each mouse received the injection in only one ear, with the other ear serving as a control.
The treatment led to better survival of hair cells and improved long-term hearing in the treated ear of both sets of mice. The earlier intervention led to greater improvements. In contrast, untreated ears developed hearing loss as expected.
The scientists also found evidence that the intervention was safe. AAV didn’t integrate into the genome of the cells it infected, which could lead to unexpected and harmful outcomes. The researchers note that additional preclinical studies will be needed in different animal models before this type of approach could be tested in humans.
“We restored the animals’ hearing, and this was sustained for at least nine months,” Chen says. “Now, we have these results that show new possibilities for genome editing. These advances are bringing in a new era of treatments for people who have genetic deafness.”
Funding: NIH’s Common Fund Somatic Cell Genome Editing program, National Center for Advancing Translational Sciences (NCATS), National Institute of Neurological Disorders and Stroke (NINDS), National Institute on Deafness and other Communications Disorders (NIDCD), and National Human Genome Research Institute (NHGRI).








