Role in mitochondrial metabolism paints more complete picture of MCL-1 function
The life and death of cells are governed by processes that—when disrupted—can lead to cancer. Apoptosis, the process of programmed cell death, is tightly regulated by the B-cell lymphoma-2 (BCL-2) family of proteins. This includes myeloid cell leukemia-1 (MCL-1), an anti-apoptotic protein that helps cells stay alive.
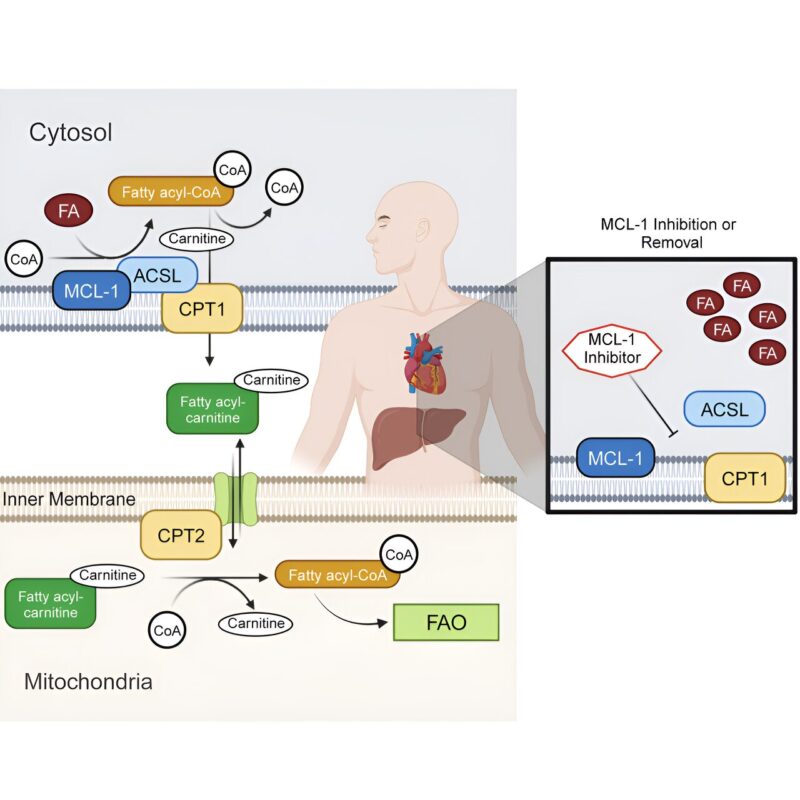
However, researchers from St. Jude Children’s Research Hospital have uncovered another critical role of MCL-1: regulating the process of long-chain fatty acid oxidation in mitochondria. These findings, published today in Molecular Cell, point to a secondary role for a well-known pro-survival protein while offering insight into confounding clinical trial results.
Proteins are often imagined as tools in a toolbox, each serving a specific function. But like modern multitools that offer an array of uses—cutting, sanding, grinding and scraping as needed—proteins often possess the ability to perform multiple functions in the cell. BCL-2 proteins are primarily associated with the tight-knit processes of keeping cells alive or sentencing them to death. Despite this association, they have been linked to other processes outside of apoptosis, none more so than MCL-1.
“It’s been amazing to us how many different cell types require MCL-1 for their everyday survival,” said corresponding author Joseph Opferman, Ph.D., St. Jude Department of Cell & Molecular Biology. MCL-1 is more than just a cell-survival workhorse; it’s also a protein with untapped therapeutic potential. “What’s made it such an important candidate for therapeutic design is it’s among the most frequently amplified genes in human cancer,” Opferman said.
MCL-1’s day job may explain clinical trial struggles
However, this therapeutic potential has come up short in clinical trials. In clinical trials, inexplicable cardiac toxicity led multiple pharmaceutical companies to either proceed with extreme caution or stop developing MCL-1 inhibitors. Opferman suspected this likely had to do with reports of the protein displaying non-apoptotic functions within the mitochondria, especially during fatty acid metabolism, but evidence for this was just as confounding.
“We’ve had a long-term interest in trying to understand the everyday job of MCL-1, things that it does in normal, healthy cells when it’s not just preventing cell death,” Opferman said. “There is more to MCL-1 than meets the eye. The protein seemed to have critical functions in regulating mitochondrial biology, and we wanted to explore this in appropriate cells.”
Our metabolism, fueled by food, produces energy for muscles. However, when our bodies don’t get enough food, they turn to their fat reserves to make up for this. The lipids are trafficked to the liver, where they are used for energy production. The researchers traced the fate of this fat in mouse models as a telltale indication that the removal of MCL-1 function disrupted metabolic function.
“It was simple to visualize that the livers had a massive accumulation of lipid droplets in them,” Opferman explained. “We hypothesized that this could be a defect in the ability to utilize those lipids. They were getting to the liver just fine but weren’t being consumed as one might expect.”

Link identified between MCL-1 and fatty acid oxidation
This led the researchers, including the study’s co-first authors Tristen Wright, St. Jude Graduate School of Biomedical Sciences, and Meghan Turnis McGehee, Ph.D., Department of Cell & Molecular Biology, to biochemically test whether there were shortcomings in lipid oxidation, ultimately discovering that the livers ability to oxidize long-chain fatty acids was defective, with no effect on short-chain fatty acids. Long-chain fatty acids make up most of the fatty acids in our bodies and are vital energy sources as well as important signaling molecules. This finding spurred the researchers on.
“We were eager to explore the biological mechanism driving this,” said Opferman. “Why was it long-chain specific?”
The missing link appeared in the form of long-chain acyl-CoA synthetase 1 (ACSL1), a protein that directs long-chain fatty acids towards the process of oxidation and which binds to MCL-1. To the researchers’ surprise, the binding between MCL-1/ACSL1 occurred at the exact same interface regulating MCL-1’s anti-apoptotic functions.
“We initially made a mutant version of MCL-1 in an attempt to enhance its interaction with non-apoptotic proteins such as ACSL1. We were shocked when the mutant MCL-1 disrupted binding with ACSL1,” said Wright. “This initial discovery allowed us to map the interaction to MCL-1’s binding pocket.”
This offered a potential mechanistic explanation for the shortcomings of clinical trials for MCL-1 inhibitors: Molecules designed to inhibit MCL-1’s cell survival functions also blocked its fatty acid oxidation functions.
“We went through many different tests to convince ourselves that that was real,” said Opferman. “I still find it one of the most amazing pieces of data we collected.”
Not giving up on MCL-1 clinical potential
Turning to the heart, the researchers found that removing MCL-1 function also disrupted long-chain fatty acid oxidation. Additionally, loss of MCL-1 lowered cellular ATP levels, the energy currency of cells. The effects were consistent whether MCL-1 was removed or inhibited.
These findings offer much-needed insight into a previously underappreciated MCL-1 function and demonstrate that BCL2 family proteins are likely involved in cell regulation outside apoptosis. While the potential of MCL-1 targeting from a therapeutic standpoint is unclear, the researchers are optimistic that further understanding of the drug mechanism may offer a route forward.
“The inhibitors are not performing well in clinical trials thus far, but in vitro, they’re very potent,” Opferman said. “We are looking into how drugs directly induce apoptosis versus this impact on fatty acid oxidation. That’s one of the things we’re interested in exploring.”
More information:
Tristen Wright et al, Anti-apoptotic MCL-1 promotes long-chain fatty acid oxidation through interaction with ACSL1, Molecular Cell (2024). DOI: 10.1016/j.molcel.2024.02.035
Citation:
Role in mitochondrial metabolism paints more complete picture of MCL-1 function (2024, March 18)
retrieved 18 March 2024
from https://medicalxpress.com/news/2024-03-role-mitochondrial-metabolism-picture-mcl.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.








