Self-adjusting brain pacemaker may help reduce Parkinson’s disease symptoms
News Release
Tuesday, August 20, 2024
Small NIH-funded trial shows the promise of personalized medicine.
A small feasibility study funded by the National Institutes of Health (NIH) found that an implanted device regulated by the body’s brain activity could provide continual and improved treatment for the symptoms of Parkinson’s disease (PD) in certain people with the disorder. This type of treatment, called adaptive deep brain stimulation (aDBS), is an improvement on a technique that has been used for PD and other brain disorders for many years. The study found aDBS was markedly more effective at controlling PD symptoms compared to conventional DBS treatments.
“This study marks a big step forward towards developing a DBS system that adapts to what the individual patient needs at a given time,” said Megan Frankowski, Ph.D., program director for NIH’s Brain Research Through Advancing Innovative Neurotechnologies® Initiative, or The BRAIN Initiative®, which helped fund this project. “By helping to control residual symptoms while not exacerbating others, adaptive DBS has the potential to improve the quality of life for some people living with Parkinson’s disease.”
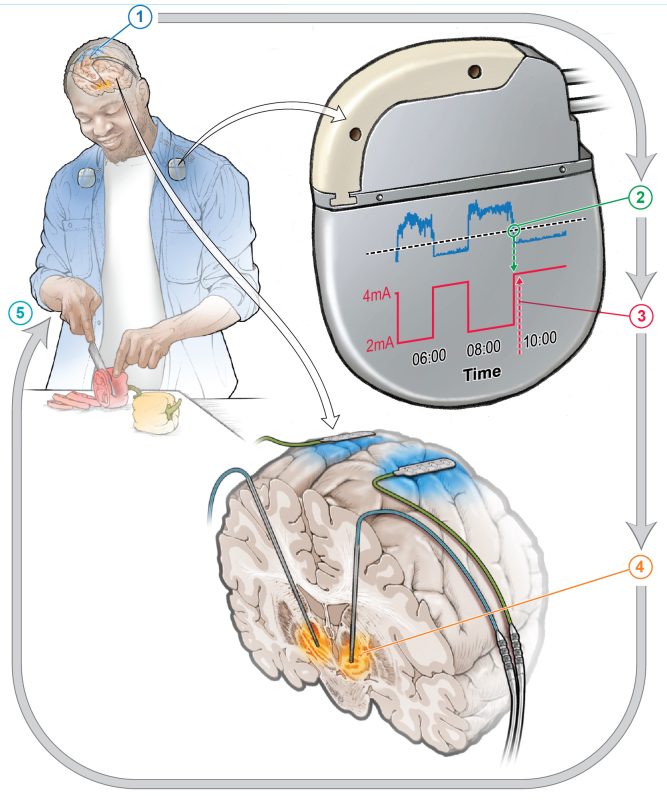
DBS involves implanting fine wires called electrodes into the brain at specific locations. These wires then deliver electrical signals that can help mitigate the symptoms of brain disorders such as PD. Conventional DBS provides a constant level of stimulation and can also lead to unwanted side effects, because the brain does not always need the same strength of treatment. Therefore, aDBS uses data taken directly from a person’s brain and uses machine learning to adjust the level of stimulation in real time as the person’s needs change over time.
Four people already receiving conventional DBS were first asked what they felt was their most bothersome symptom that had persisted despite treatment. In many instances this was either involuntary movements or difficulty in initiating movement. The participants were then set up to receive aDBS treatment alongside their existing DBS therapy. After training the aDBS algorithm for several months, the participants were sent home, where the comparison test was performed by alternating between conventional and aDBS treatments. Changes occurred every two to seven days.
aDBS improved each participant’s most bothersome symptom roughly 50% compared to conventional DBS. Notably, even though they were not told which type of treatment they were receiving at any one time, three of the four participants were often able to correctly guess when they were on aDBS due to noticeable symptom improvement.
This project is a continuation of several years of work led by Philip Starr, M.D., Ph.D., and colleagues at the University of California, San Francisco. Previously, in 2018, they reported the development of an adaptive DBS system, referred to as a “closed loop” system, that adjusted based on feedback from the brain itself. Later, in 2021, they described their ability to record brain activity in people as they went about their daily lives.
Here, those two findings were combined to use brain activity recorded during normal life activities to drive the aDBS system. However, DBS treatment changed brain activity so much that the signal that had been expected to control the aDBS system was no longer detectable. This required researchers to take a computational and data-driven approach to identify a different signal within the brains of people with PD who were receiving conventional DBS therapy.
Conventional treatment for Parkinson’s disease often involves the drug levodopa, which is used to replace dopamine in the brain that has been lost because of the disorder. Because the amount of the drug in the brain fluctuates, peaking shortly after administration of the drug and gradually decreasing as it is metabolized by the body, aDBS could help smooth out the fluctuations by providing increased stimulation when drug levels are high and vice versa, making it an attractive option for patients requiring high doses of levodopa.
While these findings are promising, there remain significant challenges to overcome for this therapy to be more widely available. The initial setup of the device requires considerable input from highly trained clinicians. Researchers envision a future where most of the work would be managed by the device itself, greatly reducing the need for repeat visits to the clinic for fine tuning.
This type of automation is also necessary for other groups to test and eventually offer aDBS therapy in a clinical setting.
“One of the big issues facing DBS, even in approved indications like Parkinson’s, is access, both for patients in terms of where they can get it and also the physicians who need special training to program these devices,” said Frankowski. “If there were a way for a system to find the most optimal settings at the press of a button, that would really increase the availability of this treatment for more people.”
This study was supported by NINDS and NIH’s The BRAIN Initiative (NS10054, NS129627, NS080680, NS120037, NS131405, NS113637), Thiemann Foundation, and the TUYF Charitable Trust Fund.
About the National Institute of Neurological Disorders and Stroke (NINDS): NINDS is the nation’s leading funder of research on the brain and nervous system. The mission of NINDS is to seek fundamental knowledge about the brain and nervous system and to use that knowledge to reduce the burden of neurological disease.
NIH’s The BRAIN Initiative, a multidisciplinary collaboration across 10 NIH Institutes and Centers, is uniquely positioned for cross-cutting discoveries in neuroscience to revolutionize our understanding of the human brain. By accelerating the development and application of innovative neurotechnologies, The BRAIN Initiative® is enabling researchers to understand the brain at unprecedented levels of detail in both health and disease, improving how we treat, prevent, and cure brain disorders. The BRAIN Initiative involves a multidisciplinary network of federal and non-federal partners whose missions and current research portfolios complement the goals of The BRAIN Initiative.
About the National Institutes of Health (NIH):
NIH, the nation’s medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit www.nih.gov.
NIH…Turning Discovery Into Health®
Article
Oehrn CR, Cernera S, Hammer LH, et al. “Chronic adaptive deep brain stimulation is superior to conventional stimulation in Parkinson’s disease: a blinded randomized feasibility trial.” Nature Medicine August 19, 2024. DOI: 10.1038/s41591-024-03196-z